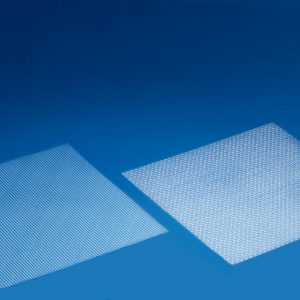
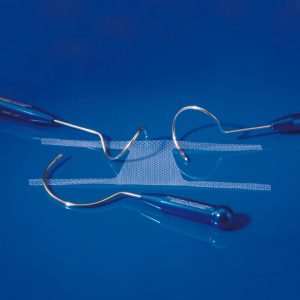
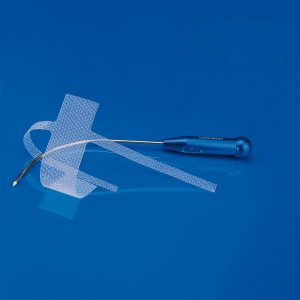
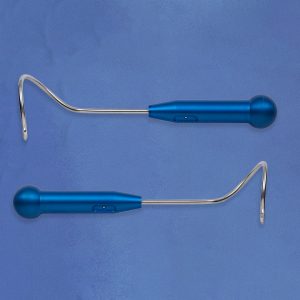
Female urinary incontinence products and pelvic floor prolapse
The pages you are about to visit include technical information about Herniamesh® products. In compliance with the guidelines of the Ministry of Health of 17 February 2010 and subsequent of 28/03/2013 and 20/12/2017, this information is exclusively for specialised medical operators. The user is fully responsible for consulting the following pages.
Read more
The products described in this section are medical devices. Medical personnel must determine in advance the suitability of use of these products for a given patient. As with any medical device, there may be complications related to the use of these products. Therefore, before using the products, medical personnel should carefully read the warnings, possible side effects, and contraindications written in the instruction booklet included in the packaging of each product, along with all relevant medical literature. Medical personnel should inform their or their patients of all potential risks associated with the use of the product.
Patients who are considering undergoing a procedure involving the implantation of a surgical prosthesis to treat urinary incontinence should be aware of the possible adverse events associated with these procedures, which are not limited to:
• bleeding including hemorrhage or hematoma, bruises, edema, seroma, abscess or fistula formation, or scarring which may occur following the implant procedure;
• urinary retention and other voiding dysfunctions (i.e. temporary or permanent urethral obstruction, urge /frequency syndrome, urge incontinence). These conditions may be associated with overcorrection/too much tension placed on the implant;
• perforations or lacerations of vessels, nerves, bladder, bowel, urethra, or any viscera, which may occur during the implantation procedure;
• extrusion through vaginal epithelium or erosion/ exposure (dehiscence) into surrounding viscera and/or mucosa;
• neuromuscular problems, foreign body sensation including acute and/or chronic pain in the groin, thigh, leg, pelvic and/or abdominal area;
• local irritation, inflammation, infection, sensitization, dyspareunia, muscular contraction;
• device migration and failure of the procedure resulting in recurrence of incontinence;
• atypical vaginal discharge, often linked to a vaginal erosion;
• detrusor muscle instability.
Please be aware that adverse events are not limited to those listed above. Always ask your physician for complete clinical data on this type of surgery.
Patients who are considering undergoing a procedure involving the implantation of a surgical prosthesis to treat pelvic floor prolapse should be aware of the possible adverse events associated with these procedures, which are not limited to:
• hematoma, seroma, abscess or fistula formation, scarring which may occur following the implant procedure;
• urinary retention and other voiding dysfunctions. These conditions may be associated with overcorrection/too much tension placed on the implant;
• perforations or lacerations of vessels, nerves, bladder, bowel, urethra, or any viscera, which may occur during the implantation procedure;
• extrusion through vaginal epithelium or erosion into surrounding viscera and/or mucosa;
• inflammation, infection, sensitization, acute and/or chronic pain, dyspareunia, contraction,
device migration and failure of the procedure resulting in recurrence of prolapse and incontinence.
Please be aware that adverse events are not limited to those listed above.
Always ask your physician for complete clinical data on this type of surgery.